Our Motivation
Pain in the lower back and other symptoms are some of the most prevalent health problems experienced by the populations of developed nations. In SODALITE, we are addressing this issue with a use-case, as these symptoms lead not only to significant losses in the quality of life of those directly affected, but also to enormous losses in productivity and costs for ongoing medical care for the entire society (see e.g. [1]).
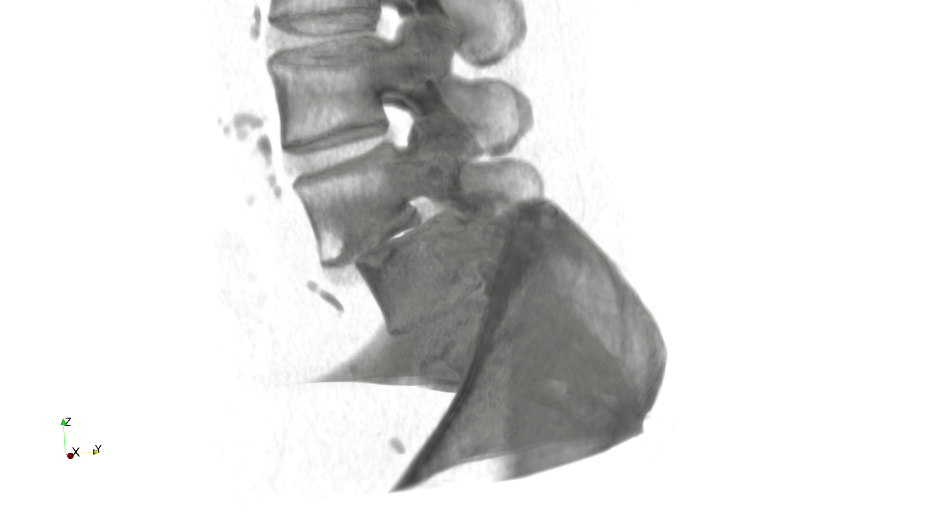
An example of a symptom that causes pain in the lower back induced by the dislocation of the fourth and fifth vertebra is shown in fig. 1.
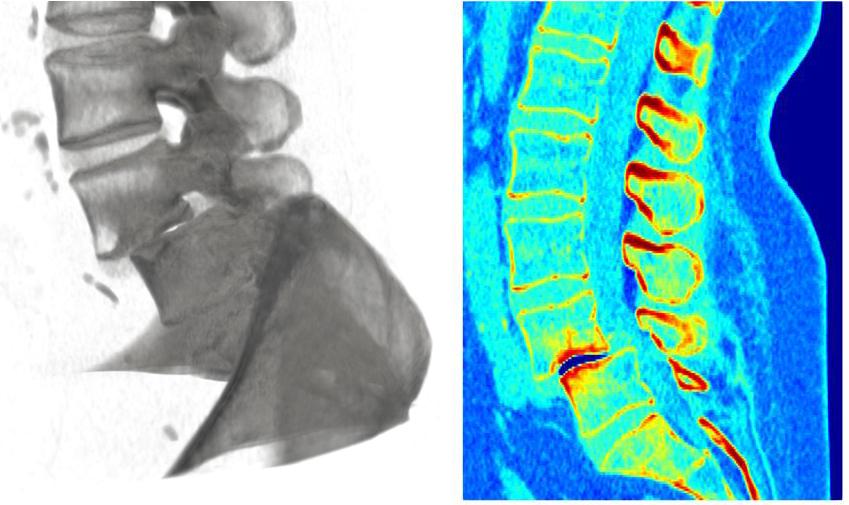
As presented in fig. 2, the treatment involves a surgical procedure which is called “spinal fusion”. In this surgery, two or more vertebrae are joined together by fixating the vertebrae against each other across the intervertebral disk by means of an implant.
The development as well as the application of implants in orthopaedic surgery is based on empirical procedures until now. Even though this leads to durable implants, complications arise in a non-negligible number of cases (c.f. e.g. [1]).
During the past years, there has been significant effort from various groups all around the world to develop the simulation of bone-implant-systems towards a stage where it can be beneficial as a clinical decision support method as well as in implant development to improve its performance and reduce complication rates. Even though biomechanical simulation methods have been lifted to a mature state, the deterministic character of numerical results is a persisting issue in their application, which foils the natural variance of biological systems. To overcome this issue, researchers propose the implementation of so called virtual clinical trials (c.f. [2]) which will allow the assessment of bone-implant-systems by means of computer simulations.
Our Ambition
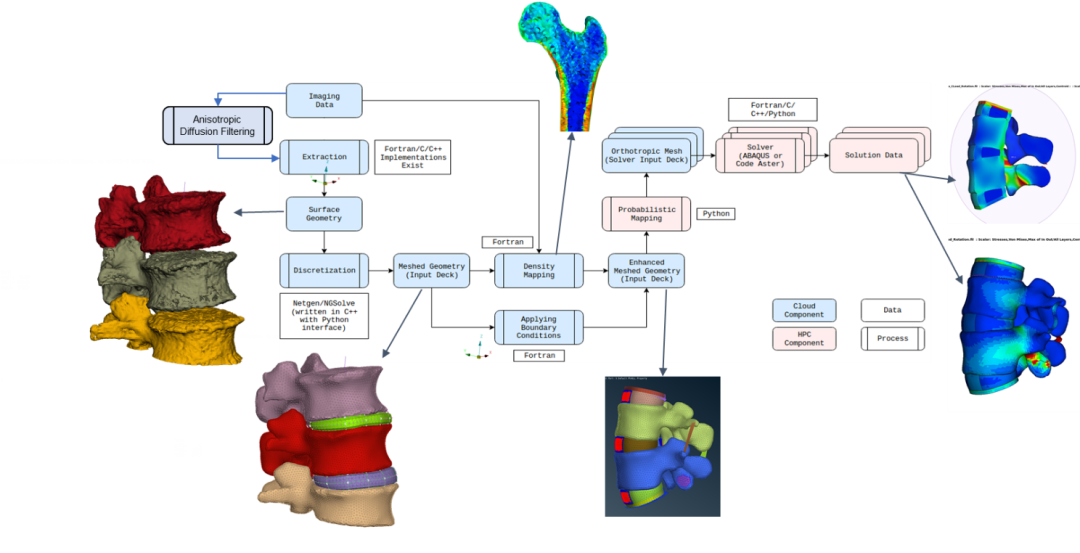
In SODALITE, we target the development of a simulation process chain supporting in-silico clinical trials of bone-implant-systems in Neurosurgery, Orthopedics and Osteosynthesis. It deals with the analysis and assessment of screw-rod fixation systems for instrumented mono- and bi-segmental fusion of the lumbar spine by means of continuum mechanical simulation methods. As a novelty, we will consider the uncertainty inherent in the computation by means of probabilistic programming. The simulation chain consists of a number of steps that need to be fulfilled in order and can be considered a pipeline. The output of each step serves as input to the next step as shown in fig. 3.
The developed simulation process will help to optimise screw-rod fixation systems based on clinical imaging data recorded during standard examinations and consequently target the lowering of the reported rates of screw loosening and revisions, enhance safety, expand the knowledge of the internal mechanics of screw-rod fixation systems applied to the lumbar spine and finally reveal optimisation potential in terms of device application and design. Once established, the proposed method will make the treatment outcome and development of implants for orthopaedic surgery also more robust, since doctors can recognise irregularities in the healing process much earlier.
Benefits of SODALITE for the use-case
This use case expects significant benefits from the SODALITE project. Since the methodology of in-silico clinical trials in biomechanical simulations stands at the border of being productively used in medical device development, it becomes especially important to increase the effectiveness of deployment, management and adaption to different IT-infrastructures. This will ease the use of the methodology for end users like medical device manufacturers or medical research institutes and it will additionally significantly reduce the support effort of the developers. In view of the productive and with that commercial application of in-silico clinical trials, besides efficient deployment and development methodologies, performance optimisations both static as well as dynamic ones are definitely required. This is due to the requirement of in-silico clinical trials to apply simulation process chains to complete patient cohorts which will consume a significant amount of computing time and thus a significant amount of money. This means, the targeted use-case will significantly benefit from the developments of SODALITE both in terms of turn-around times of the complete simulation procedure as well as economically.
The impact of the developments we expect to achieve within SODALITE to the broader community is manifold but will mainly affect the fields of health research, eHealth and the engineering services domain. The methodologies used throughout the implementation and integration on top of SODALITE will serve as a major breakthrough for future simulation-based services. A successful implementation facilitating a cohort size comparable to the ones used in classical clinical trials will demonstrate that it is indeed possible to lift the academic methodology, of analysing biomechanical devices by means of continuum mechanical simulation approaches, to a state in which it will not only be a beneficial tool in health research but also in biomechanical device development and design. It will serve as a demonstrator for a wide spectrum of bone-implant systems. Evaluation studies of the implemented storage solution, simulation process chain and data analytics strategies will furthermore be of significant value for the continued designed and development of personalised medicine.
[1] International Society for the Advancement of Spine Surgery, “Policy Statement – Lumbar Spinal Fusion,” https://www.isass.org/public-policy/isass-policy-statement-on-lumbar-spinal-fusion-surgery/
[2] The Avicenna Roadmap: “in silico Clinical Trials: How Computer Simulation will Transform the Biomedical Industry” Coordination and Support Action funded by EC as part of the 7th Framework Program Duration: October 2013 - September 2015